

Kite pharma how to#
Once he did, however, Belldegrun knew how to market its potential to investors.
Kite pharma plus#
They include Agensys, which he sold to Astella for $537 million in 2007, and Cougar, sold to Johnson & Johnson for $1 billion.Īcquiring the rights to eACt was a complicated affair, involving four sellers: the two developers plus the commercialization arms of Weizmann and the NIH. While Belldegrun is a professor at the University of California Los Angles and has wide medical interests, he is first and foremost an entrepreneur, founding and selling three startups in the past decade. Belldegrun bought the rights to the technology and built Kite around it.Ī Weizmann and Hebrew University Medical School graduate, the 67-year-old Belldegrun moved to Los Angeles at the start of his career and met Rosenberg when they were both researchers at the NIH.

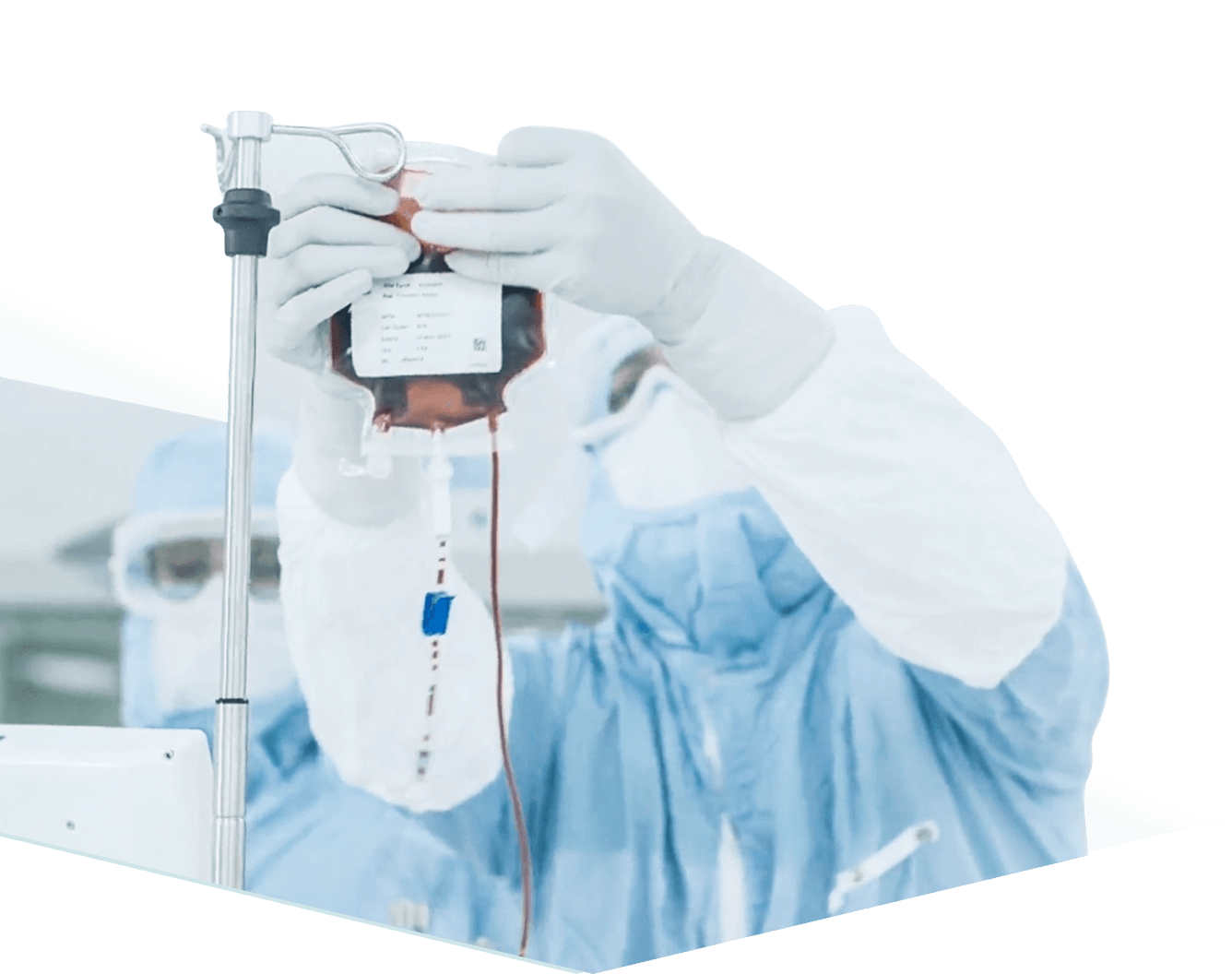
Kite’s core technology, called eACT, was developed by Weizmann Institute of Science immunologist Prof. The gap was quickly closed with Kite shares soaring 28 percent in Nasdaq trading to $178.24. That came to $180 a share, a 29 percent premium on Kite’s share price even after a strong run this year. Gilead said it had agreed to pay $11.9 billion in cash for Kite, which is based in Santa Monica, California and is developing immunotherapy-based cancer treatments. Arie Belldegrun, and his ability to leverage a vast store of medical knowledge and Wall Street connections. The almost $12 billion that Gilead Sciences agreed on Monday to pay for Kite Pharma is testament not only to Israeli innovation but also to the entrepreneurship of Kite’s Israeli founder and CEO, Prof. Adimab enables its partners to rapidly expand their biologics pipelines through a broad array of technology access arrangements.The headquarters of Gilead Sciences in Foster City, California Credit: Eric Risberg, AP Gilead Sciences agreed to 29 percent premium, which shrank fast in pretrading, despite biotech company Kite Pharma’s low revenues, heavy R&D spending, and losses Adimab offers fundamental advantages by delivering diverse panels of therapeutically relevant antibodies and bispecifics that meet the most aggressive standards for affinity, epitope coverage, species cross-reactivity and developability. About AdimabĪdimab’s integrated antibody discovery and optimization platform provides unprecedented speed from antigen to purified, full-length human lgGs. In addition, Adimab has transferred its platform for broad use to GSK, Biogen and Novo Nordisk. Adimab has also partnered with many mid-size and early-stage venture-backed companies, including Merrimack, Five Prime, Jounce, Innovent, Alector, Acceleron, Oncothyreon, Surface Oncology, Potenza, Arsanis and others. Adimab’s funded discovery partners include many leading pharmaceutical companies, such as Merck, Roche, Novartis, Eli Lilly, Genentech, Gilead, Kyowa Hakko Kirin, Sanofi and others. Over the past 5+ years, Adimab has established funded discovery collaborations with over 30 companies.
Kite pharma license#
Adimab will receive an undisclosed upfront payment, research fees and technical milestones, as well as license fees, certain additional milestones and royalties on any therapeutic CAR product sales that are optioned under the agreement. Kite will also have the option to exclusively license antibodies for development and commercialization as therapeutic CAR products. For each target, the agreement grants Kite the right to research antibodies generated during the collaboration for potential use in therapeutic CAR-T products. Kite and Adimab have entered into a collaboration whereby Adimab will use its proprietary discovery and optimization platform to identify fully human antibodies against selected targets. “Kite is focused on finding partnerships, such as this collaboration with Adimab, that help us establish a competitive advantage in the CAR-T space,” said Margo Roberts, Ph.D., Chief Scientific Officer of Kite.
“Our platform can rapidly deliver a diverse panel of antibodies with varying affinities and ultra-high selectivity.” “Kite has established itself as a leader in the field of engineered autologous cell therapies, and we are excited to have the opportunity to work with them,” said Tillman Gerngross, Chief Executive Officer and co-founder of Adimab. Under the terms of the agreement, Adimab will use its proprietary platform to generate IgGs against multiple targets selected by Kite. Adimab, LLC, the technology leader in the discovery of fully human antibodies and bispecifics, today announced a new collaboration with Kite Pharma, Inc., (Nasdaq:KITE).


 0 kommentar(er)
0 kommentar(er)
